Exploring Methane as a Rocket Propellant
Fueling the Future of Space Exploration
Space Trivia
What was the propellant used in the first human-made object to reach outer space?
A) Liquid Hydrogen and Liquid Oxygen
B) Liquid Oxygen and Ethyl Alcohol (Ethanol)
C) Hypergolic Propellants
D) Solid Rocket Boosters
Answers at the end.
A private company in China has launched the world's first methane-liquid oxygen rocket. The move helps China beat rivals like the US, India, and Europe in developing the next-generation launch vehicle that could carry payloads into orbit around Earth. The Zhuque-2 carrier rocket blasted off from the Jiuquan Satellite Launch Center in northwest China.
The race to explore the cosmos has led scientists and engineers to continually seek more efficient and sustainable fuel sources for rocket propulsion. In recent years, methane has emerged as a promising candidate, offering a range of advantages that could revolutionize space exploration. This article delves into the characteristics of methane as a rocket fuel, its production methods, and its potential applications in advancing our reach into the universe.
The Chemistry of Methane
Methane, a simple hydrocarbon composed of one carbon atom and four hydrogen atoms (CH₄), stands out for its energy density and clean combustion. In comparison to traditional rocket propellants like kerosene, methane releases more energy per unit mass, making it an attractive option for rocketry. Additionally, its combustion results in carbon dioxide and water, minimizing the production of environmentally harmful byproducts.
Production Methods
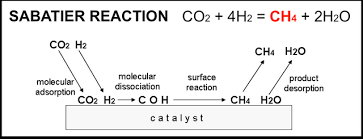
Methane can be produced through various methods, each with its own set of advantages and challenges. One common approach is the Sabatier reaction, which involves the reaction of carbon dioxide and hydrogen. This process can be particularly advantageous in space missions where carbon dioxide is readily available, such as on Mars. Another method involves extracting methane from natural gas deposits or synthesizing it from biomass, providing flexibility in sourcing.
Benefits for Space Exploration
Energy Density: Methane's high energy density allows rockets to carry more payload while requiring less fuel mass. It has a higher specific impulse, which means one kg of the gas can lift one kg of mass for a longer time.This is crucial for extended space missions where efficiency is paramount.
In-Situ Resource Utilization (ISRU): Methane's potential for production from local resources, such as the carbon dioxide in Mars' atmosphere, aligns with the concept of In-Situ Resource Utilization. This could reduce the need to transport fuel from Earth, lowering mission costs.
Reusable Rockets: Methane's cleaner combustion facilitates the development of reusable rocket technologies. Rockets powered by methane can undergo more cycles of use without significant degradation, contributing to a more sustainable approach to space travel.
Mars Colonization: The prospect of methane production on Mars is particularly significant for future colonization efforts. Using local resources for fuel production could be a game-changer in establishing sustainable human habitats on the Red Planet.
Challenges and Mitigations
Cryogenic Storage: While methane's cryogenic storage requirements are less demanding than hydrogen, they still pose challenges. Advances in insulation technologies and materials aim to address these challenges, ensuring the efficient storage of methane over extended mission durations.
Engine Development: The development of methane-powered rocket engines necessitates engineering modifications due to differences in combustion characteristics compared to traditional fuels. Ongoing research aims to optimize engine designs for methane, enhancing performance and reliability.
Infrastructure Transition: The aerospace industry's transition to methane as a primary rocket fuel requires significant infrastructure changes. Launch facilities, ground support equipment, and spacecraft systems need adaptation to accommodate methane-based propulsion systems.
Starter Mechanism: A methane-powered rocket, also known as Methalox, uses methane as the fuel and liquid oxygen (LOX) as the oxidizer. It operates based on the same principles as traditional launch vehicles but differs in terms of propellant choice and certain design considerations. But one major disadvantage is that methane-fired engines need an igniter to start the fire whereas Hydrazine fuels are hypergolic, which means they start burning on their own upon coming in contact with oxygen.
Several missions have embraced methane as a fuel, SpaceX's ambitious Starship spacecraft utilizes liquid methane and liquid oxygen for its Raptor engines. Blue Origin's BE-4 rocket engine, designed for its New Glenn rocket, employs liquid methane. This engine signifies methane's viability for large-scale launch vehicles.
But Wednesday's launch put China ahead of its US rivals, including Elon Musk's SpaceX and Jeff Bezos' Blue Origin, in the race to launch carrier vehicles fueled by methane. As the aerospace industry continues to innovate, methane stands as a beacon, guiding us toward sustainable and efficient space exploration.
Regarding the Space trivia question.
Correct answer: B) Liquid Oxygen and Ethyl Alcohol (Ethanol)
The German A-4 ballistic rocket of World War II (better known by its propaganda name V-2), which is credited as having begun the space age, used ethanol as the main constituent.




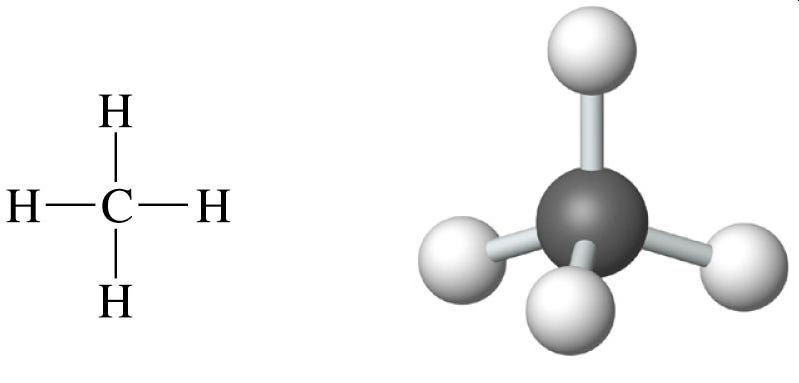
What I love about SpaceX is its ability to embrace the counterintuitive. In the search for the most efficient rocket fuel, hydrogen appears to be the winner hands down, with the highest possible energy-to-mass ratio.
BUT, because methane has a higher density than hydrogen, it requires a physically smaller rocket to contain that energy. Hydrogen must also be kept extremely cold, requiring a lot more thermal management/insulation. Hydrogen is known to be leaky and make metals brittle.
When looking at the entire rocket as a system, methane is actually the most efficient fuel. SpaceX was the first to notice this, now the rest of the industry is running to catch up.